+44 (0)1588 620192
info@productapprovals.co.uk
Recently added item(s) ×
You have no items in your shopping basket.
UKCA - Certification
|
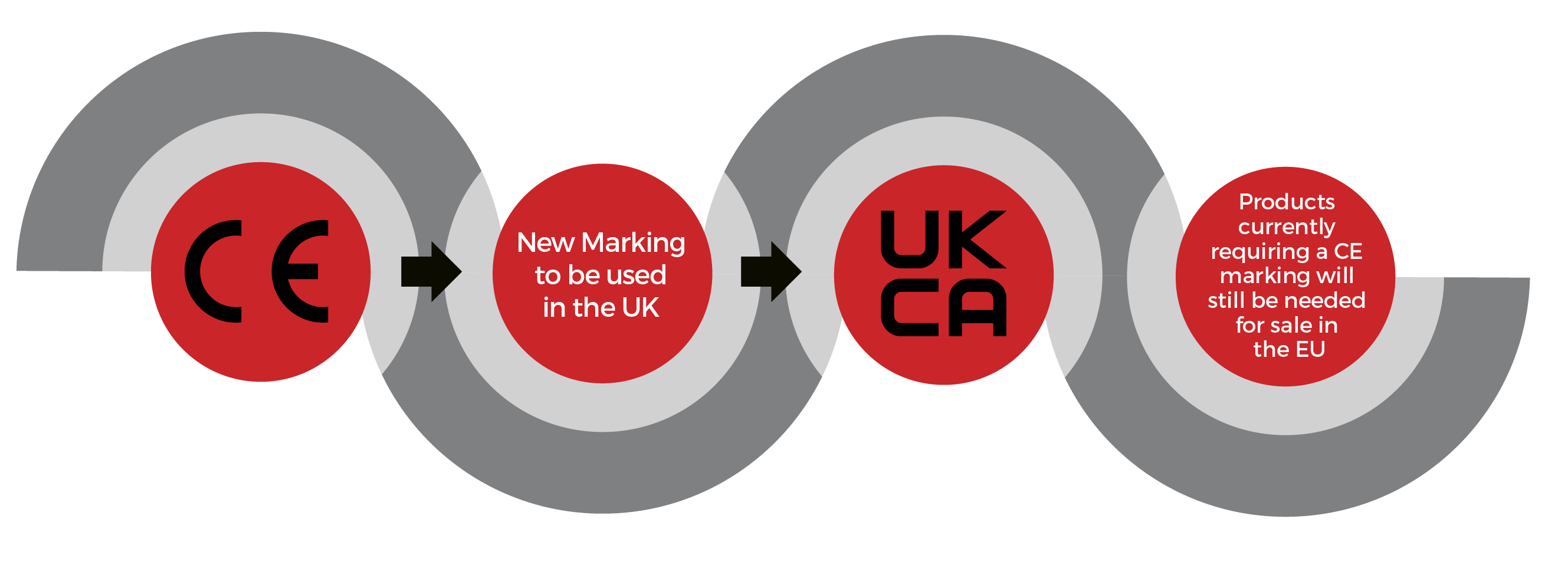
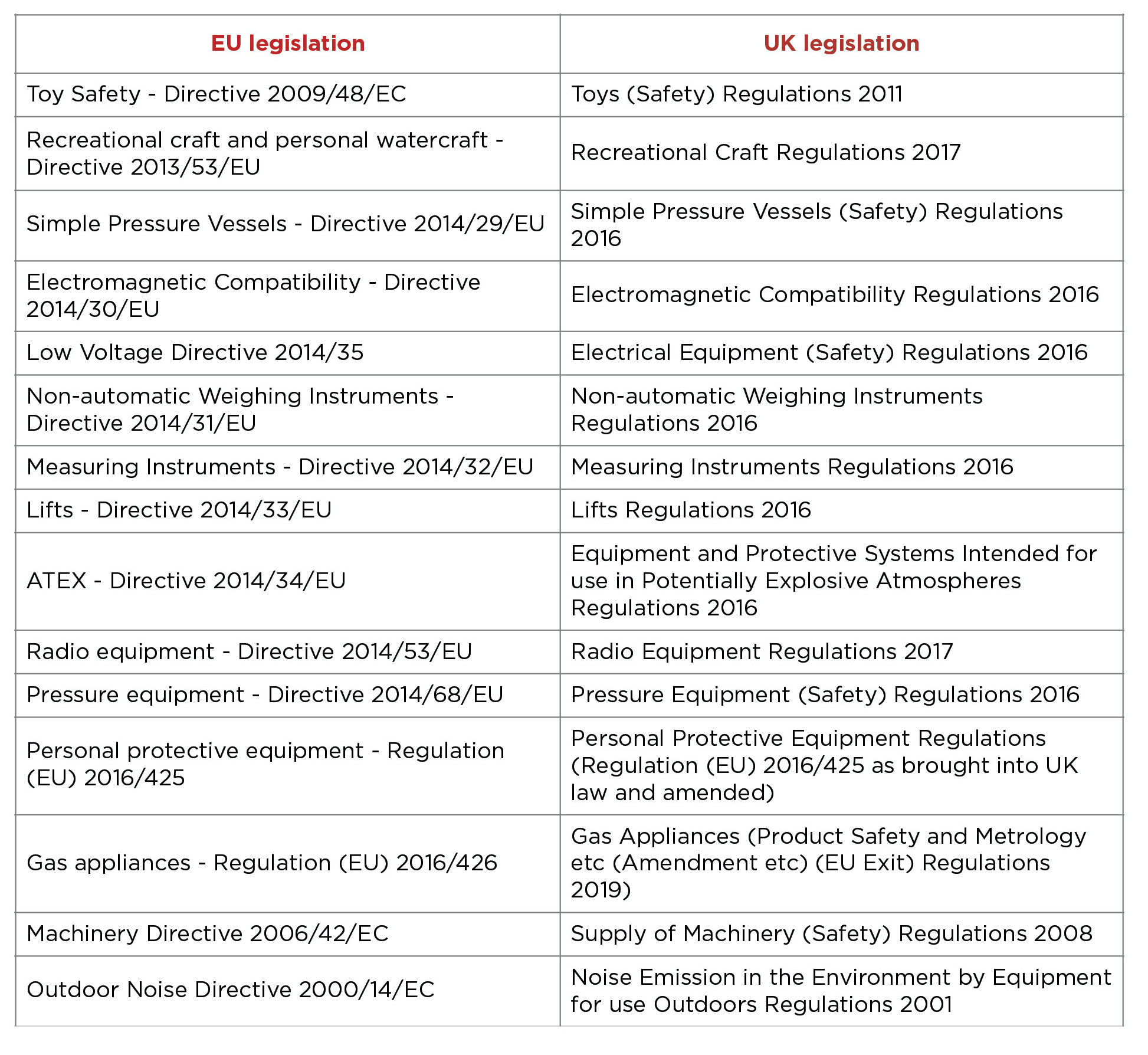
UKCA (UK Conformity Assessment) is the product marking system intended to replace CE marking for the GB market (England, Wales and Scotland). For most goods being placed on the GB market, the UKCA mark implementation date has been extended, indefinitely, beyond December 2024.UKCA mark ExtensionCurrently, the UKCA mark is accepted on the Great Britain market but the Government announced an indefinite extension Different rules apply for several product categories including medical. For the full list, see the below Government link: https://www.gov.uk/guidance/using-the-ukca-marking Northern Ireland UKCA Marking will apply throughout GB and all products will need to have UKCA or CE marking to be traded in England, Wales, or Scotland. At the end of the transition period, the Ireland / Northern Ireland (IE/NI) protocol applies which means that goods placed on the NI market are subject to EU law, so therefore require CE marking. This means that when necessary products will need to have had their Notified Body conformity Assessments updated by an EU notified Body to maintain the CE Marking. Products that have been certified by a NI Notified Body will need to mark the product next to the CE mark with UK(NI). This distinct marking allows the identification of products which can be legally placed on the market in NI but not the EU. Products marked with UK(NI) will be allowed to be sold in the UK with no additional regulatory checks. Authorised RepresentativeAn entity who first places the product on the UK market becomes a UK importer. An entity who first places the product on the EU market becomes an EU importer. These entities assume the associated responsibilities of an importer. When selling products into Europe you will need an EU address for the product, so either the importer puts their address on the packaging and therefore assumes responsibility or you can use an EU authorised representative office service. UKCA requirements will mirror the CE marking rules which means that the manufacturer’s name and address must be on the product, packaging, or associated documentation. If the manufacturer is NOT established in the UK, then the name and address of the UK importer must also be on the product, packaging, or associated documentation. The manufacturer may also use a UK authorised representative where their details need to be on the product, packaging, or associated documentation. This information is the current interpretation as of September 2023 – Changes may take place which affect the below guidance. DOWNLOAD E-FLYER
|
|